NIH initiates Phase 1 trial of universal flu vaccine candidate April 1, 2025, enrolling 24 healthy volunteers aged 18-50 for two intramuscular injections.
HHS launches $500 million initiative for universal influenza vaccine, aiming for 2029 FDA approval with human clinical trials starting 2026.
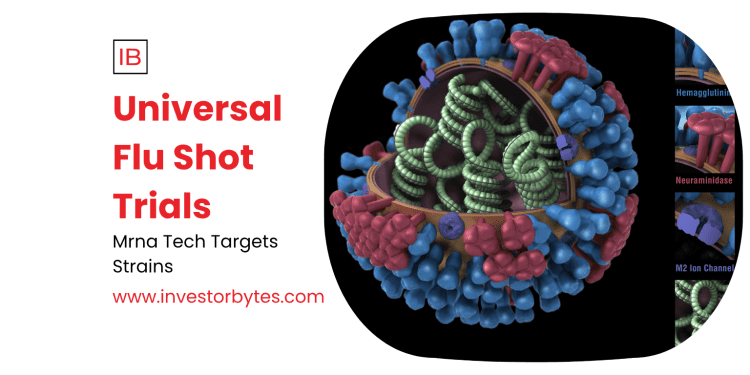
Broad-spectrum strategy targets conserved viral parts, protecting against all influenza strains, NIAID Vaccine Research Center leading Phase 1/2 studies.
The project addresses seasonal flu’s 290,000-650,000 annual deaths, developing mRNA or viral vector platforms for lasting immunity.
Early trials test safety, immunogenicity in adults, Phase 2/3 to follow for efficacy across age groups and variants.
Partners include BARDA, CEPI for global access, $100 million Phase 1 funding to accelerate development.
This trial’s quiet innovation unveils new era where universal protection bridges seasonal gaps, transforming flu defense with enduring harmony.